Physical Chemistry
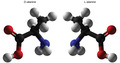
(View Complete Item Description)Physical Chemistry is the application of physical principles and measurements to understand the properties of matter, as well as for the development of new technologies for the environment, energy and medicine. Advanced Physical Chemistry topics include different spectroscopic methods (Raman, ultrafast and mass spectroscopy, nuclear magnetic and electron paramagnetic resonance, x-ray absorption and atomic force microscopy) as well as theoretical and computational tools to provide atomic-level understanding for applications such as: nanodevices for bio-detection and receptors, interfacial chemistry of catalysis and implants, electron and proton transfer, protein function, photosynthesis and airborne particles in the atmosphere.
Material Type: Textbook