Kitchen Science: Whole Wheat Soda Bread with Butter and Chai – Chemical Reactions: Chemical Leaveners in Baking
Summary
In this 8th grade science lesson, students prepare Whole Wheat Soda Bread with fresh churned butter and Chai. While the bread bakes in the oven, students experiment with chemical leaveners and observe the chemical reaction that causes the Whole Wheat Soda Bread to rise.
Objectives
After this lesson, students will be able to:
- Use cabbage juice as an indicator to determine whether a kitchen ingredient is acidic, basic, or neutral
- Identify characteristics of chemical and physical changes
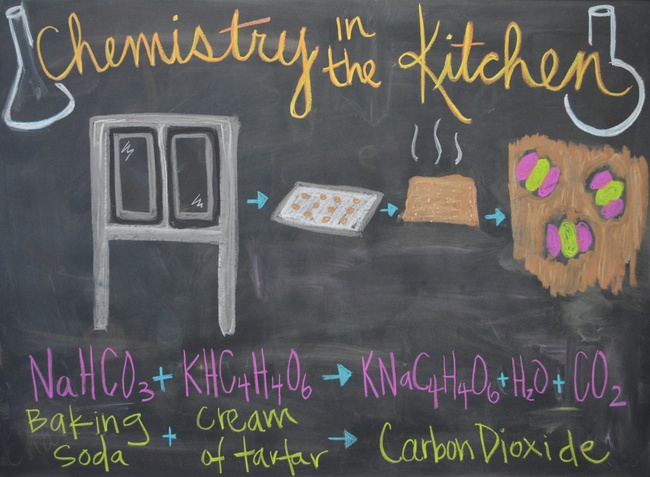
- Recognize that baking powder, when combined with liquid ingredients, produces a chemical reaction that releases CO2 (carbon dioxide) and causes bread to rise
Assessments
During this lesson, students will:
- Use cabbage juice as an indicator to test whether a solution is acidic, basic, or neutral
- Make observations and look for evidence of chemical and physical changes
- Identify the chemical reaction that creates CO2 and causes bread to rise
Materials for the Chef Meeting
- Whole Wheat Soda Bread recipe
- Chai recipe
- Ingredients and tools for demonstration
- Visual aid
Ingredients
For the Whole Wheat Soda Bread
- Whole wheat flour
- Sugar
- Salt
- Baking soda
- Baking powder
- Egg
- Butter
- Buttermilk
For the Butter
- Heavy cream or whipping cream
For the Chai
- Water
- Ginger
- Pepper
- Cardamom pods
- Milk
- Sugar
- Decaffeinated black tea
Tools
For the Whole Wheat Soda Bread
- Mixing bowls
- Measuring cups
- Measuring spoons
- Whisk
- Wooden spoon
- Sheet pan lined with parchment paper
For the Butter
- Butter churn or mason jar (with a lid)
- Small stone or marble
For the Chai
- Stock pot
- Measuring cups
- Measuring spoons
- Wooden spoon
Equipment
- Oven
- Stove
For the Chemical Leaveners Lab
- 4 clear cups labeled:
- Baking Soda (NaHCO3)
- Cream of Tartar (KH4H4O6)
- Baking Soda + Cream of Tartar → CO2
- Baking Powder
- Cabbage juice indicator
- Water
- Cream of tartar
- Baking soda
- Measuring spoons
- Baking powder
- Measuring cups
- Spoons for stirring
- Visual aid of the pH scale
Before You Begin
- Make the cabbage juice indicator
- Create the visual aid
- Copy the Whole Wheat Soda Bread recipe to hand out
- Copy the Chai recipe to hand out
Procedures
At the Chef Meeting
- Welcome the students and introduce the Whole Wheat Soda Bread with butter and Chai. Explain that this is the first part of a two-part lesson that focuses on chemical changes in baking that cause bread to rise. Today, we will be using a chemical leavener (baking powder), and for the next lesson we will use a biological leavener (yeast).
- Define leavener and explain that leaveners (both chemical and biological) make bread rise by producing gas (CO2). With both chemical and biological leaveners, the production of CO2 is caused by a chemical reaction.
- Invite students to define a physical change and explain the making butter is an example of a physical change. When shaken or churned, the cream (a liquid) will turn into butter (a solid).
- Students should know that a physical change is any change in appearance (size, shape, or state) of matter that does not change the identity of the substance. Melting, boiling and dissolving are physical changes, and physical changes are reversible.
- Invite students explain a chemical reaction in comparison it to a physical change.
- Students should know that a chemical reaction produces substances that are different from the starting substances. In a chemical reaction, the atoms rearrange to form a brand new substance (i.e., baking soda + cream of tartar → CO2). One clue that a chemical reaction is occurring is the formation of bubbles, which indicates that gas is being produced.
- Explain that since chemical reactions occur on a molecular level, it is more difficult to see. However, we can use our senses to collect evidence and make observations that will reveal whether a change is chemical:
- Sight: Did the physical properties change?
- Touch: Is there a change in temperature?
- Sound: Is gas being produced?
- Taste: Does it taste different?
- Smell: Does it smell different?
- Ask students to wash their hands and join their table group.
At the Table
- Meet with the table groups to review the recipe and assign jobs.
- Prepare the recipes and, once the bread is in the oven, gather students around the table for the Chemical Leaveners Lab.
- Set the table.
- Eat.
- Clean up.
At the Chemical Leaveners Lab
- Pour ¼ cup of cabbage juice indicator into the cup labeled Baking Soda (NaHCO3) and the cup labeled Cream of Tartar (KH4H4O6).
- Measure out 1 teaspoon of baking soda and stir it into the cup labeled Baking Soda (NaHCO3). Observe for color change and have the students approximate a number on the pH scale for the baking soda (it will be basic).
- Measure out 2 teaspoons of cream of tartar and stir it into the cup labeled Cream of Tartar (KH4H4O6). Observe for color change and have the students approximate a number on the pH scale for the cream of tarter (it will be acidic).
- Place 1 teaspoon of baking soda and 2 teaspoons of cream of tarter into the cup labeled Baking Soda + Cream of Tartar → CO2. Pour in ¼ cup of water and observe the reaction and look for evidence of chemical change.
- Combine the cups labeled Baking Soda (NaHCO3) and Cream of Tartar (KH4H4O6). Have students observe the reaction and look for evidence of chemical change (the chemical change is the same but this time the cabbage juice will indicate that the mixture has gone back towards neutral).
- Explain that baking powder is made up of baking soda and cream of tartar. Using their observations from the previous three cups, have students hypothesize the chemical reaction and color that will occur when you cabbage juice to the baking powder.
- Add ¼ cup of cabbage juice to the cup labeled Baking Powder. Add 1 tablespoon of baking powder to the cabbage juice and have the students compare the cream of tartar and baking soda solution to the baking powder solution.
- Explain how baking powder makes bread rise by producing CO2 (point out that this is what is currently happening in the oven). Because baking powder needs a liquid medium in which to react, we keep the liquid ingredients and the dry ingredients separate until the end of the recipe so that the chemical reaction the produces CO2 occurs right before we put our bread into the oven.
- Emphasize that both baking soda and cream of tartar taste harsh and, if unbalanced, can leave behind a chemically flavor in whatever you are baking.
At the Closing Circle
Identify a physical change or chemical change that is prevalent in cooking.