EVAPORATION
Evaporation
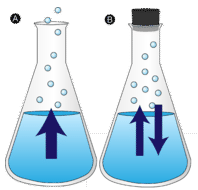
A puddle of water left undisturbed eventually disappears. The liquid molecules escape into the gas phase, becoming water vapor. Vaporization is the process in which a liquid is converted to a gas. Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid. If the water is instead kept in a closed container, the water vapor molecules do not have a chance to escape into the surroundings and so the water level does not change. As some water molecules become vapor, an equal number of water vapor molecules condense back into the liquid state. Condensation is the change of state from a gas to a liquid.
In order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Recall that a given liquid sample will have molecules with a wide range of kinetic energies. Liquid molecules that have this certain threshold kinetic energy escape the surface and become vapor. As a result, the liquid molecules that remain now have lower kinetic energy. As evaporation occurs, the temperature of the remaining liquid decreases. You have observed the effects of evaporative cooling. On a hot day, the water molecules in your perspiration absorb body heat and evaporate from the surface of your skin. The evaporating process leaves the remaining perspiration cooler, which in turn absorbs more heat from your body.
A given liquid will evaporate more quickly when it is heated. This is because the heating process results in a greater fraction of the liquid's molecules having the necessary kinetic energy to escape the surface of the liquid. The figure below shows the kinetic energy distribution of liquid molecules at two temperatures. The numbers of molecules that have the required kinetic energy to evaporate are shown in the shaded area under the curve at the right. The higher temperature liquid has more molecules that are capable of escaping into the vapor phase than the lower temperature liquid .

Summary
- Evaporation is the conversion of a liquid to its vapor below the boiling temperature of the liquid.
- Condensation is the change of state from a gas to a liquid.
- As the temperature increases, the rate of evaporation increases.
https://www.youtube.com/watch?v=WQ8_yF5PI5A
REFERENCES
From chemistry/Libretexts, source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/13%3A_States_of_Matter/13.08%3A_Evaporation