Lewis Structures
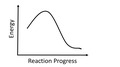
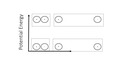
(View Complete Item Description)This multi-part module introduces covalent bonding and Lewis structures as a model of covalent bonding.Starting with valence electrons, a method of connecting unpaired electrons and/or redistributing valence electrons to satisfy the octet rule is introduced.Numerous examples are presented including CO, ozone, and polyatomic ions
Material Type: Module