BASIC THERMODYNAMICS
Thermodynamics is the science that deals with the interaction between energy and
material systems.
DEFINITIONS
SYSTEM
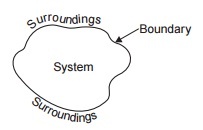
A system is a finite quantity of matter or a prescribed region of space.
SURROUNDING
Everything external to the system is called surroundings or the environment.
BOUNDARY
The actual or hypothetical envelope enclosing the system is the boundary of
the system. The boundary may be fixed or it may move, as and when a system containing a gas is
compressed or expanded. The boundary may be real or imaginary.
TYPES OF SYSTEM
CLOSED SYSTEM
If the boundary of the system is impervious to the flow of matter, it is
called a closed system. An example of this system is mass of gas or vapour contained in an engine
cylinder, the boundary of which is drawn by the cylinder walls, the cylinder head and piston
crown. Here the boundary is continuous and no matter may enter or leave.
OPEN SYSTEM
An open system is one in which matter flows into or out of the system.
Most of the engineering systems are open
ISOLATED SYSTEM
An isolated system is that system which exchanges neither energy nor matter with any
other system or with environment.
HOMOGENEOUS SYSTEM
A system which consists of a single phase is termed as homogeneous system. Examples :
Mixture of air and water vapour, water plus nitric acid and octane plus heptane.
ADIABATIC SYSTEM
An adiabatic system is one which is thermally insulated from its surroundings. It can,
however, exchange work with its surroundings. If it does not, it becomes an isolated system.
HETERGENEOUS SYSTEM
A system which consists of two or more phases is called a heterogeneous system. Examples :
Water plus steam, ice plus water and water plus oil.
THERMODYNAMIC EQUILIBRIUM
A system is in thermodynamic equilibrium if the temperature and pressure at all points
are same ; there should be no velocity gradient ; the chemical equilibrium is also necessary.
Systems under temperature and pressure equilibrium but not under chemical equilibrium are
sometimes said to be in metastable equilibrium conditions. It is only under thermodynamic equilibrium
conditions that the properties of a system can be fixed.
Thus for attaining a state of thermodynamic equilibrium the following three types of equilibrium
states must be achieved :
THERMAL EQUILIBRIUM
The temperature of the system does not change with time and
has same value at all points of the system.
CHEMICAL EQUILIBRIUM
No chemical reaction takes place in the system and the chemical
composition which is same throughout the system does not vary with time.
MECHANICAL EQUILIBRIUM
There are no unbalanced forces within the system or between
the surroundings. The pressure in the system is same at all points and does not change with
respect to time.
References
1. Engineering Thermodynamics by P K Nag
2.Engineering Thermodynamics by R K Rajput