TREATMENT METHODOLOGY
The hard water can be softened by the following methods:
*Ion Exchange Process
*Lime Soda Process
*Reverse Osmosis Process
Ion Exchange Process:
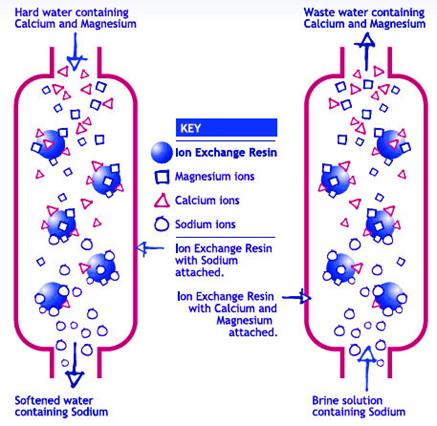
Calcium (Ca2+) and magnesium (Mg2+) ions that cause water hardness can be removed fairly easily by using an ion exchange procedure. Standard water softeners are cation exchange devices. Cations refer to positively charged ions dissolved in the water. Cation exchange involves the replacement of the hardness ions with a nonhardness ion.
Water softeners usually use sodium (Na+) as the exchange ion. Sodium ions are supplied from dissolved sodium chloride salt, also called brine. In the ion exchange process, sodium ions are used to coat an exchange medium in the softener. The exchange medium can be natural “zeolites” or synthetic resin beads that resemble wet sand.
As hard water passes through a softener, the calcium and magnesium trade places with sodium ions (Figure 1). Sodium ions are held loosely and are replaced easily by calcium and magnesium ions. During this process, “free” sodium ions are released into the water.
After softening a large quantity of hard water, the exchange medium becomes coated with calcium and magnesium ions. When this occurs, the exchange medium must be recharged or regenerated.To recharge the softener with sodium ions, a softener is backflushed with a salt brine solution. During a backflush, the brine solution replaces the calcium and magnesium ions on the exchange medium with sodium ions from the salt solution.
Lime Soda Process
Soda lime is a process used in water treatment to remove Hardness from water. This process is now obsolete but was very useful for the treatment of large volumes of hard water. Addition of lime (CaO) and soda (Na2CO3) to the hard water precipitates calcium as the carbonate, and magnesium as its hydroxide. The amounts of the two chemicals required are easily calculated from the analysis of the water and stoichiometry of the reactions. The lime‐soda uses lime, Ca (OH)2 and soda ash, Na2CO3, to precipitate hardness from solution.
Carbon dioxide and carbonate hardness (calcium and Magnesium bicarbonate) are complexed by lime. In this process Calcium and Magnesium ions are precipitated by the addition of lime (Ca(OH)2) and soda ash (Na2CO3).
Following are the reactions that takes place in this process:
As slacked lime is added to a water, it will react with any carbon dioxide present as follows
Ca(OH)2 + CO2 → CaCO3 ↓ + H2O....(1)
The lime will react with carbonate hardness as follows:
Ca(OH)2 + Ca(HCO3 )2 → 2CaCO3 ↓ + 2H2O.....(2)
Ca(OH)2 + Mg(HCO3 )2 → MgCO3 + CaCO3 ↓ + 2H2O.....(3)
The product magnesium carbonate in equation 3 is soluble. To remove it, more lime is added:
Ca(OH)2 + MgCO3 → CaCO3↓ + Mg(OH)2↓.....(4)
Also, magnesium non-carbonate hardness, such as magnesium sulfate, is removed:
Ca(OH)2 + MgSO4 → CaSO4 + Mg(OH)2↓.....(5)
Lime addition removes only magnesium hardness and calcium carbonate hardnes In equation 5 magnesium is precipitated, however, an equivalent amount of calcium is added. The water now contains the original calcium non-carbonate hardness and the calcium non-carbonate hardness produced in equation 5. Soda ash is added to remove calcium non-carbonate hardness:
Na2CO3 + CaSO4 → Na2SO4 + CaCO3↓.....(6)
To precipitate CaCO3 requires a pH of about 9.5; and to precipitate Mg(OH)2 requires a pH of about 10.8, therefore, an excess lime of about 1.25 meq/l is required to raise the pH.
The amount of lime required: lime (meq/l) = carbon dioxide (meq/l) + carbonate hardness (meq/l) + magnesium ion (meq/l) + 1.25 (meq/l)
The amount of soda ash required: soda ash (meq/l) = non-carbonate hardness (meq/l)
After softening, the water will have high pH and contain the excess lime and the magnesium hydroxide and the calcium carbonate that did not precipitate. Recarbonation (adding carbon dioxide) is used to stabilize the water. The excess lime and magnesium hydroxide are stabilized by adding carbon dioxide, which also reduces pH from 10.8 to 9.5 as the following:
CO2 + Ca(OH)2 → CaCO3↓ + H2O
CO2 + Mg(OH)2 → MgCO3 + H2O
Further recarbonation, will bring the pH to about 8.5 and stablize the calcium carbonate as the following:
CO2 + CaCO3 + H2O → Ca(HCO3)2
It is not possible to remove all of the hardness from water. In actual practice, about 50 to 80 mg/l will remain as a residual hardness.

Reverse Osmosis Process:
Reverse Osmosis is the process of Osmosis in reverse. Whereas Osmosis occurs naturally without energy required, to reverse the process of osmosis you need to apply energy to the more saline solution. A reverse osmosis membrane is a semi-permeable membrane that allows the passage of water molecules but not the majority of dissolved salts, organics, bacteria and pyrogens. However, you need to 'push' the water through the reverse osmosis membrane by applying pressure that is greater than the naturally occurring osmotic pressure in order to desalinate (demineralize or deionize) water in the process, allowing pure water through while holding back a majority of contaminants.
Below is a diagram outlining the process of Reverse Osmosis. When pressure is applied to the concentrated solution, the water molecules are forced through the semi-permeable membrane and the contaminants are not allowed through.

Reverse Osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi-permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
The desalinated water that is demineralized or deionized, is called permeate (or product) water. The water stream that carries the concentrated contaminants that did not pass through the RO membrane is called the reject (or concentrate) stream.
A short video presentation of Reverse Osmosis