
13 Results

Chemistry is the study of matter, and all matter is made up of atoms. We will learn about elements, atomic number and mass, isotopes, moles (chemistry moles, not the animal), and compounds.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Lesson
- Provider:
- Khan Academy
- Provider Set:
- Khan Academy
- Date Added:
- 06/26/2019

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.
- Subject:
- Biology
- Life Science
- Material Type:
- Full Course
- Provider:
- Rice University
- Provider Set:
- OpenStax College
- Date Added:
- 08/22/2012
- Subject:
- Biology
- Life Science
- Material Type:
- Unit of Study
- Provider:
- Rice University
- Provider Set:
- OpenStax College
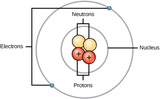
By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Author:
- Tina B. Jones
- Date Added:
- 08/26/2019

By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

Students learn about the periodic table and how pervasive the elements are in our daily lives. After reviewing the table organization and facts about the first 20 elements, they play an element identification game. They also learn that engineers incorporate these elements into the design of new products and processes. Acting as computer and animation engineers, students creatively express their new knowledge by creating a superhero character based on of the elements they now know so well. They will then pair with another superhero and create a dynamic duo out of the two elements, which will represent a molecule.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- TeachEngineering
- Provider Set:
- TeachEngineering
- Author:
- Brian Kay
- Denise W. Carlson
- Lauren Cooper
- Malinda Schaefer Zarske
- Megan Podlogar
- Date Added:
- 10/14/2015

In this course, we will explore what makes things in the world the way they are and why, to understand the science and consider the engineering. We learn not only why the physical world behaves the way it does, but also how to think with chemical intuition, which can’t be gained simply by observing the macroscopic world.
This 2018 version of 3.091 by Jeffrey Grossman and the 2010 OCW version by Don Sadoway cover similar topics and both provide complete learning materials. This 2018 version also includes Jeffrey Grossman’s innovative Goodie Bags, Why This Matters, and CHEMATLAS content, as well as additional practice problems, quizzes, and exams.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Full Course
- Provider Set:
- MIT OpenCourseWare
- Author:
- Grossman, Jeffrey
- Date Added:
- 09/01/2018

Through three lessons and their four associated activities, students are introduced to concepts related to mixtures and solutions. Students consider how mixtures and solutions and atoms and molecules can influence new technologies developed by engineers. To begin, students explore the fundamentals of atoms and their structures. The building blocks of matter (protons, electrons, neutrons) are covered in detail. The next lesson examines the properties of elements and the periodic table one method of organization for the elements. The concepts of physical and chemical properties are also reviewed. Finally, the last lesson introduces the properties of mixtures and solutions. A comparison of different mixtures and solutions, their properties and their separation qualities are explored.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Full Course
- Provider:
- TeachEngineering
- Provider Set:
- TeachEngineering
- Date Added:
- 10/14/2015
Hank does his best to convince us that chemistry is not torture, but is instead the amazing and beautiful science of stuff. Chemistry can tell us how three tiny particles - the proton, neutron, and electron - come together in trillions of combinations to form ... everything. In this inaugural episode of Crash Course Chemistry, we start out with one of the biggest ideas in chemistry ever - stuff is made from atoms. More specifically, we learn about the properties of the nucleus and why they are important to defining what an atom actually is.
Chapters:
Intro
Einstein & Atoms
Composition of Atoms
Atomic Number
Isotopes
Relative Atomic Mass
Mass Number
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Lecture
- Provider:
- Complexly
- Provider Set:
- Crash Course Chemistry
- Date Added:
- 02/24/2013
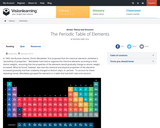
This module introduces D. Mendeleev's theories and the modern Periodic Table. Electron configuration as a function of table placement is discussed.
- Subject:
- Astronomy
- Chemistry
- Education
- Physical Science
- Space Science
- Material Type:
- Interactive
- Unit of Study
- Provider:
- UCAR Staff
- Provider Set:
- Visionlearning
- Author:
- Anthony Carpi
- Date Added:
- 03/18/2003
This lesson plan examines the properties of elements and the periodic table. Students learn the basic definition of an element and the 18 elements that build most of the matter in the universe. The periodic table is described as one method of organization for the elements. The concepts of physical and chemical properties are also reviewed.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Activity/Lab
- Lesson Plan
- Provider:
- TeachEngineering
- Provider Set:
- TeachEngineering
- Author:
- Brian Kay
- Daria Kotys-Schwartz
- Janet Yowell
- Malinda Schaefer Zarske
- Date Added:
- 09/18/2014