- Author:
- Anna McCollum, ALka Sharma, Jillian Gorrell, Amanda Spangler, Madonna Kemp
- Subject:
- Agriculture, Life Science
- Material Type:
- Textbook
- Level:
- High School, Community College / Lower Division
- Tags:
- License:
- Creative Commons Attribution Non-Commercial No Derivatives
- Language:
- English
3.3 Nutrient Availability
3.4 Cation Exchange Capacity
3_Soil-Chemical-Properties
Soil Chemical Properties
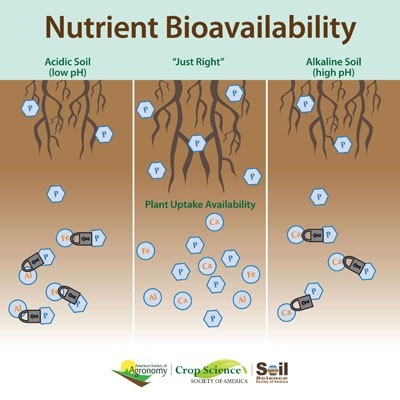
Overview
Title Image "Nutrient bioavailability with regards to soil pH" is copyrighted and used with permission from the American Society of Agronomy, Crop Science Society of America and Soil Science Society of America.
Did you have an idea for improving this content? We’d love your input.
Introduction
Learning Objectives
Explain the chemical properties of the soil and soil/medium pH on nutrient availability.
Explain how soil pH effect nutrient availability for plants.
Describe the process of cation exchange.
Explain how negatively charged mineral ions are more likely to be leached.
Key Terms
acidic soil - soil with a pH level less than 7
alkaline soil - soil with a pH level greater than 7
anion - negative ion that is formed by an atom gaining one or more electrons
cation - positive ion that is formed by an atom losing one or more electrons
cation exchange capacity - the measure of the total amount of exchangeable positive ions that a soil can hold
ion - atom or chemical group that does not contain equal numbers of protons and electrons
leach - the act of chemicals or minerals being drained away from soil by water
soil electrical conductivity - measure of the amount of salts in soil
Soil pH
Even though most plants are autotrophs and can generate their own sugars from carbon dioxide and water, they still require certain ions and minerals from the soil. An ion is an atom or chemical that does not contain equal numbers of protons and electrons. Ions are either anions or cations. An anion is a negative ion that is formed by an atom gaining one or more electrons, and a cation is a positive ion that is formed by an atom losing one or more electrons. By definition, “pH” is a measure of the active hydrogen ion (H+) concentration. It is an indication of the acidity or alkalinity of a soil, and is also known as “soil reaction.” The pH scale ranges from 0 to 14, with values below 7.0 being considered acidic and values above 7.0 alkaline. A pH value of 7 is considered neutral, where H+ and OH- are equal, both at a concentration of 10-7 moles/liter. A pH of 4.0 is ten times more acidic than a pH of 5.0. Some minor elements (e.g., iron) and most heavy metals are more soluble at lower pH. This makes pH management important in controlling movement of heavy metals (and potential groundwater contamination) in soil.
The most important effect of pH in the soil is on ion solubility, which in turn affects microbial and plant growth. A pH range of 6.0 to 6.8 is ideal for most crops because it coincides with optimum solubility of the most important plant nutrients. Not all ions are equally available in soil water; their availability depends on the properties of the soil. Clay is negatively charged; thus, any positive ions (cations) present in clay-rich soils will remain tightly bound to the clay particles. This tight association with clay particles prevents the cations from being washed away by heavy rains, but it also prevents the cations from being easily absorbed by plant root hairs. In contrast, anions are easily dissolved in soil water and thus readily accessible to plant root hairs; however, they are also very easily leached or washed away by rainwater. In this way, the presence of clay particles creates a trade-off for plants: they prevent leaching of cations from the soil by rainwater, but they also prevent absorption of the cations by the plant. In acid soils, hydrogen and aluminum are the dominant exchangeable cations. The latter is soluble under acidic conditions, and its reactivity with water (hydrolysis) produces hydrogen ions. Calcium and magnesium are basic cations; as their amounts increase, the relative amount of acidic cations will decrease. Let's take phosphorous as an example (Figure 4.2.1) If soils are too acidic, phosphorus reacts with iron and aluminum. That makes it unavailable to plants. But if soils are too alkaline, phosphorus reacts with calcium and also becomes inaccessible.
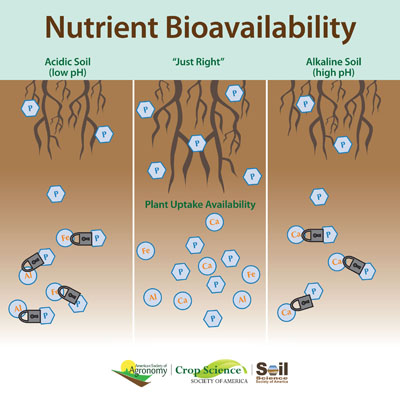
Factors that affect soil pH include parent material, vegetation, and climate. Some rocks and sediments produce soils that are more acidic than others: quartz-rich sandstone is acidic; limestone is alkaline. Some types of vegetation, particularly conifers, produce organic acids, which can contribute to lower soil pH values. In humid areas such as the eastern US, soils tend to become more acidic over time because rainfall washes away basic cations and replaces them with hydrogen. Addition of certain fertilizers to soil can also produce hydrogen ions. Liming the soil adds calcium, which replaces exchangeable and solution H+ and raises soil pH. Lime requirement, or the amount of liming material needed to raise the soil pH to a certain level, increases with CEC. To decrease the soil pH, sulfur can be added, which produces sulfuric acid.
Nutrient Availability
How do plants acquire micronutrients from the soil? This process is mediated by root hairs, which are extensions of the root epidermal tissue that increase the surface area of the root, greatly contributing to the absorption of water and minerals. Root hairs absorb ions that are dissolved in the water in soil.
How do plants overcome these issues?
The cell in the root utilizes active transport (use of energy to transport a substrate against the concentration gradient) to move mineral ions into the root cells. Proton pumps or ATPases, use ATP as an energy source to pump protons out of the cells and into the soils leading to an increase in the concentration of protons (H+) thus lowering the pH or acidifying the microscopic area of soil surrounding the root hair and generating electrochemical gradient (difference in concentration and electrical charge of species across a membrane). These pumps are located on the plasma membrane and are found in the cells of root hair, cortex, and endodermal layer. Based on apoplastic, symplastic or transmembrane route (unit 2, lesson 3 Xylem transport) the mineral ions are actively loaded into the root vascular system.
Protons pumped in the soil may participate in exchange of cations on the surface of the soil particles or accompany an anion into a plant cell or generate the gradient for specific ion to be transported across the plasma membrane into the cell. To facilitate the transport of cations and anions into the plant cells proton pumps work in conjugation with either antiporters, symporters, or uniporters (Figure 4.3.2). Antiporters transport different ions or molecules across the plasma membrane in opposite directions while symporters transport ions or molecules in the same direction. Uniporters transport one specific ion or molecule across the plasma membrane.
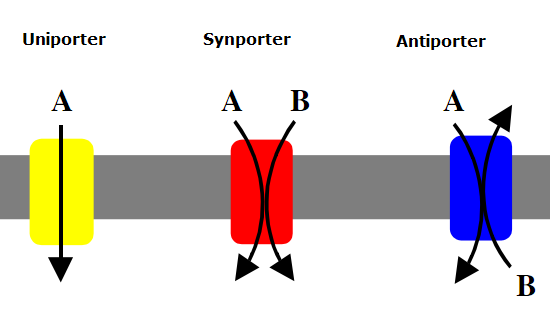
Electrochemical gradient leads to two outcomes:
- Protons bind to the negatively charged clay particles, replacing the cations from the clay in a process called cation exchange. The cations then diffuse down their electrochemical gradient into the root hairs. High concentration of negatively charged organic anions within the cells also favor the transport of cations into the cells.
- The high concentration of protons in the soil creates a strong electrochemical gradient that favors transport of protons back into the root hairs. Plants use co-transport of protons via symporters down their concentration gradient as the energy source to move anions against their electrical gradient into the root hairs. (The soil environment is highly positively charged, so it is unfavorable for anions to leave the soil, but highly favorable for protons to leave the soil).
As of now, proton pumps are considered central to the mineral ion transport across the root plasma membrane. However, studies of the involvement of redox chains, and OH- efflux transporters during anion transport are also underway. Redox chains located on the plasma membrane are utilized by many plants, such as corn and oats, for anion absorption. Redox chains pump electrons out of the cells, thus creating an electrical gradient for anion uptake. More research is in progress to complete the characterization of proteins in the redox chains and understand their mechanisms. OH-efflux transporters are unique in enhancing the anion absorption by excreting negatively charged hydroxyl ion (OH-) outside of the root cell. These transporters need further research to increase our understanding of anion absorption in plants.
Cation Exchange Capacity
The cation exchange capacity of a soil is a measurement of the magnitude of the negative charge per unit weight of soil or the amount of cations a particular sample of soil can hold in an exchangeable form. The greater the clay and organic matter content, the greater the cation exchange capacity should be; although, different types of clay minerals and organic matter can vary in cation exchange capacity. soil electrical conductivity is a measure of the amount of salt in soil. Because salts move with water; low areas, depressions, or other wet areas where water accumulates tend to be higher in electrical conductivity than surrounding higher-lying, better drained areas. Clay soils dominated by clay minerals that have a high cation-exchange capacity have higher electrical conductivity than clay soils dominated by clay minerals that have a low cation exchange capacity. Soils with restrictive layers, such as claypans, typically have higher electrical conductivity because salts cannot be leached from the root zone and accumulate on the surface.
Cation exchange is an important mechanism in soils for retaining and supplying plant nutrients, as well as for adsorbing contaminants. For example, it plays an important role in wastewater treatment in soils. Sandy soils with a low cation exchange capacity are generally unsuited for septic systems since they have little adsorptive ability and there is potential for groundwater.
Due to the influence of pH and clay on ion retention, as well as other parameters, the composition and texture of soil greatly influences the ability of roots to penetrate the soil, as well as the availability of water, nutrients, and oxygen:
Composition | Water availability | Nutrient availability | Oxygen availability | Root penetration ability |
Sand | Low: water drains out | Low: poor capacity for cation exchange; anions leach out | High: many air-containing spaces | High: large particles do not pack tightly |
Clay | High: water clings to charged surface of clay particles | High: large capacity for cation exchange; anions remain in solution | Low: few air-containing spaces | Low: small particles pack tightly |
Organic matter | High: water clings to charged surface of clay particles | High: ready source of nutrients, large capacity for cation exchange; anions remain in solution | High: many air-containing spaces | High: large particles do not pack tightly |
While plants have ready access to carbon (carbon dioxide) and water (except in dry climates or during drought), they must extract minerals and ions from the soil. Often nitrogen is most limiting for plant growth; while it comprises approximately 80% of the atmosphere, gaseous nitrogen is chemically stable and not biologically available to plants. Many plants have evolved mutualistic relationships with microorganisms, such as specific species of bacteria and fungi, to enhance their ability to acquire nitrogen and other nutrients from the soil. This relationship improves the nutrition of both the plant and the microbe.
Dig Deeper
Cation Exchange Video https://youtu.be/HmEyymGXOfI
Mineral Absorption Video: https://youtu.be/6aC-WTAWgOg
Attributions
"Adhesion and Cohesion of Water" by the United States Department of Agriculture Natural Resources Conservation Service is in the Public Domain.
Haynes, R.J. Active ion uptake and maintenance of cation-anion balance: A critical examination of their role in regulating rhizosphere pH. Plant Soil 126, 247–264 (1990). https://doi.org/10.1007/BF00012828
"Nutrient Acquisition by Plants" by Georgia Tech Biological Sciences is licensed under CC BY-NC-SA 3.0.
"Nutrient Bioavailability" graphic used with permission from the American Society of Agronomy, Crop Science Society of America and Soil Science Society of America.
OpenStax Biology 2e by Mary Ann Clark, Matthew Douglas, and Jung Choi is licensed under CC BY 4.0.
"Soil Electrical Conductivity" by the United States Department of Agriculture Natural Resources Conservation Service is in the Public Domain.
"Soil Physical and Chemical Properties" by the United States Department of Agriculture Natural Resources Conservation Service is in the Public Domain.