Learning modules that describe how to convert between number of molecules, moles and mass, for students in General Chemistry.
- Subject:
- Physical Science
- Material Type:
- Module
- Date Added:
- 07/20/2016

Learning modules that describe how to convert between number of molecules, moles and mass, for students in General Chemistry.
Language is complicated, especially in organic chemistry. This episode of Crash Course Organic Chemistry is all about nomenclature. We'll dive into IUPAC systematic naming of organic molecules, and get to practice with the help of three trusty steps!
A lot of ionic compounds dissolve in water, dissociating into individual ions. But when two ions find each other and form an insoluble compound, they suddenly fall out of the solution in what's called a precipitation reaction. In this episode of Crash Course Chemistry, we learn about precipitation, precipitates, anions, cations, and how to describe and discuss ionic reactions.
Chapters:
Precipitate Reactions
Determining Precipitates
Writing Precipitate Reactions
Calculating Molar Mass Equation
This 15-minute video lesson looks at the double Newman diagram for methcyclohexane. [Organic Chemistry playlist: Lesson 18 of 73].
This 8-minute video lesson looks at cyclic ethers and epoxide naming. [Organic Chemistry playlist: Lesson 49 of 73].
What's that smell? Smell's like Organic Chemistry! This week Hank talks about Aromatics and Cyclic Compounds, naming their substituents, resonance, and common reactions & uses.

Chemistry Behind the Magic features videos of exciting live chemistry demonstrations. The videos are enhanced by explanations of the science behind the demonstration, in a fun and easy-to-understand format.
This set of videos showcases exciting live chemistry demonstrations held at MIT. Through the magic of chemistry, Dr. John Dolhun and Dr. Bassam Shakhashiri create things that steam, fizzle, and glow. Each video also provides a deeper look into the chemistry that makes it all possible.
For teachers, we have provided supporting materials to help you understand and replicate the experiments in your own classrooms. These videos can be watched in any order.
WARNING NOTICE
The experiments described in these materials are potentially hazardous. Among other things, the experiments should include the following safety measures: a high level of safety training, special facilities and equipment, the use of proper personal protective equipment, and supervision by appropriate individuals. You bear the sole responsibility, liability, and risk for the implementation of such safety procedures and measures. MIT and Dow shall have no responsibility, liability, or risk for the content or implementation of any of the material presented. Legal Notice
Paul Anderson's video playlist of videos that can be used in a AP Chemistry Video Essentials class
Introduction to Polymers
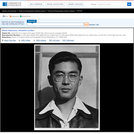
Masao Nakazawa, chemistry teacher, bust portrait, facing front. Title transcribed from Ansel Adams' caption on verso of print. Original neg. no.: LC-A35-4-M-28. Gift; Ansel Adams; 1965-1968. Forms part of: Manzanar War Relocation Center photographs.
This sample shell is produced by the California Community Colleges CVC-OEI to support faculty in the use of Open Educational Resources and development of courses aligned to the OEI Course Design Rubric. The shell may be used for online, hybrid, &/or face-to-face classes. The shell is available for all faculty, not just those faculty in the CCC system. The team producing this shell includes Helen Graves, Liezl Madrona, Cyrus Helf, Nicole Woolley & Barbara Illowsky. If you are having challenges importing the shell, here are some steps to take. (1) Create an empty shell in your sandbox. (2) Import the Canvas Commons course into your shell. (3) Adapt the content as you wish. (4) If all else fails, contact your college IT person or Canvas administrator.
Almost all Anatomy and Physiology course require the student have a basic level of chemistry knowledge. Lesson 02 Chemistry covers the basics of inorganic chemistry, organic chemistry, physics, and nutrition that most A&P student need to make it through the course.
Keep in mind that each of these topics takes about a year of college to gain a better understanding; this video series is just a quick survey to get you up to speed.

This research-based student project used the problem of ocean acidification to cover the sustainability concept of fossil fuel combustion and the disciplinary concepts of kinetics, equilibrium, acid-base chemistry and solubility.
(Note: this resource was added to OER Commons as part of a batch upload of over 2,200 records. If you notice an issue with the quality of the metadata, please let us know by using the 'report' button and we will flag it for consideration.)
This 12-minute video lesson looks at chair and boat Shapes for cyclohexane. [Organic Chemistry playlist: Lesson 17 of 73].
Short Description:
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry.
Long Description:
The goal of this textbook is not to make you an expert. True expertise in any field is a years-long endeavor. Here I will survey some of the basic topics of chemistry. This survey should give you enough knowledge to appreciate the impact of chemistry in everyday life and, if necessary, prepare you for additional instruction in chemistry. Throughout each chapter, I present two features that reinforce the theme of the textbook—that chemistry is all around you. The first is a feature titled, appropriately, “Chemistry Is Everywhere.” Chemistry Is Everywhere” focuses on the personal hygiene products that you may use every morning: toothpaste, soap, and shampoo, among others. These products are chemicals, aren’t they? Ever wonder about the chemical reactions that they undergo to give you clean and healthy teeth or shiny hair? I will explore some of these chemical reactions in future chapters. But this feature makes it clear that chemistry is, indeed, everywhere. The other feature focuses on chemistry that you likely indulge in every day: eating and drinking. In the “Food and Drink App,” I discuss how the chemistry of the chapter applies to things that you eat and drink every day. Carbonated beverages depend on the behavior of gases, foods contain acids and bases, and we actually eat certain rocks. (Can you guess which rocks without looking ahead?) Cooking, eating, drinking, and metabolism—we are involved with all these chemical processes all the time. These two features allow us to see the things we interact with every day in a new light—as chemistry.
Word Count: 183565
(Note: This resource's metadata has been created automatically as part of a bulk import process by reformatting and/or combining the information that the author initially provided. As a result, there may be errors in formatting.)
Life is chaos and the universe tends toward disorder. But why? If you think about it, there are only a few ways for things to be arranged in an organized manner, but there are nearly infinite other ways for those same things to be arranged. Simple rules of probability dictate that it's much more likely for stuff to be in one of the many disorganized states than in one of the few organized states. This tendency is so unavoidable that it's known as the 2nd Law of Thermodynamics. Obviously, disorder is a pretty big deal in the universe and that makes it a pretty big deal in chemistry - it's such a big deal that scientists have a special name for it: entropy. In chemistry, entropy is the measure of molecular randomness or disorder. For the next thirteen minutes, Hank hopes you will embrace the chaos as he teaches you about entropy.
Chapters:
Second Law of Thermodynamics
Entropy
DEMONSTRATION!
BA(OH)2•8H2O+NH4Ci
J.W. Gibbs & Gibbs Free Energy
This 7-minute video lesson shows how to represent the structures of organic molecules. [Organic Chemistry playlist: Lesson 1 of 73].
When we venture to new places, we need navigational tools to guide us. In organic chemistry, those are reaction mechanisms! In this episode of Crash Course Organic Chemistry, we’ll learn all about how to write reaction mechanisms. Having this super useful skill means we don’t have to worry about memorizing every reaction that has ever existed.

Why does chemistry matter in my life? These lessons address this question and are designed to be used throughout the high school course and support the North Carolina standard course of study objectives in chemistry. Each lesson presents a problem to the student that they will endeavor to answer using a variety of activities. These activities may be modified to suit the needs of your students. The problems are intended to generate student interest so they will be more likely to engage in the lesson.

This collection of videos, animations and documents comes from the NCSSM AP chemistry online course. Chapter three part one provides practice and demonstrations related to atomic structure in chemistry.