
Determine the enthalpy of the ATP reaction.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- Carnegie Mellon University
- Provider Set:
- The ChemCollective
- Date Added:
- 02/05/2021

Determine the enthalpy of the ATP reaction.

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.


By the end of this section, you will be able to:Define “energy”Explain the difference between kinetic and potential energyDiscuss the concepts of free energy and activation energyDescribe endergonic and exergonic reactions

By the end of this section, you will be able to:Define “energy”Explain the difference between kinetic and potential energyDiscuss the concepts of free energy and activation energyDescribe endergonic and exergonic reactions

Students act as engineers to learn about the strengths of various epoxy-amine mixtures and observe the unique characteristics of different mixtures of epoxies and hardeners. Student groups make and optimize thermosets by combining two chemicals in exacting ratios to fabricate the strongest and/or most flexible thermoset possible.
In this part of the MRE scenario, students measure the enthalpy of a reaction.
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.

Polymers are a vital part of our everyday lives and nearly all consumer products have a plastic component of some variation. Students explore the basic characteristics of polymers through the introduction of two polymer categories: thermoplastics and thermosets. During teacher demos, students observe the unique behaviors of thermoplastics. The fundamentals of thermoset polymers are discussed, preparing them to conduct the associated activity in which they create their own thermoset materials and mechanically test them. At the conclusion of this lesson-activity pair, students understand the basics of thermoplastics and thermosets, which may entice their interest in polymer engineering.
Use the virtual lab to determine how much milk to add to hot coffee to reach the desired temperature

Engineering thermodynamics is a branch of science that deals with the study of energy conversion and its relationship with heat and work. It's a fundamental discipline in engineering, providing the principles necessary for the analysis and design of various energy systems and processes. In this article, topics such as the systems, state, process, properties of pure substances, heat and work, and thermal equilibrium are meticulously explained to enable easy comprehension. This study guide would enable students to use thermodynamics to optimize the performance and efficiency of engines, power plants, refrigeration systems, and other devices that involve energy transfer. Additionally, understanding this concept of engineering thermodynamics would equip students with the initiative to design a sustainable system that helps mitigate greenhouse gases from fossil fuels.

The students discover the basics of heat transfer in this activity by constructing a constant pressure calorimeter to determine the heat of solution of potassium chloride in water. They first predict the amount of heat consumed by the reaction using analytical techniques. Then they calculate the specific heat of water using tabulated data, and use this information to predict the temperature change. Next, the students will design and build a calorimeter and then determine its specific heat. After determining the predicted heat lost to the device, students will test the heat of solution. The heat given off by the reaction can be calculated from the change in temperature of the water using an equation of heat transfer. They will compare this with the value they predicted with their calculations, and then finish by discussing the error and its sources, and identifying how to improve their design to minimize these errors.
Energy is like the bestest best friend ever and yet, most of the time we take it for granted. Hank feels bad for our friend and wants us to learn more about it so that we can understand what it's trying to tell us - like that any bond between two atoms contains energy. How much energy? That's not the simplest question to answer, but today Hank will answer it (kinda), by teaching us about a nifty little thing called enthalpy.
If you are paying attention to this episode you'll learn what the state function is, and how it varies from a path-dependent function; why enthalpy change is different from heat; that bonds are energy and to form and break them they release and absorb heat to and from their environment. You'll get the quickest introduction to calorimetry ever (more on that in upcoming episodes) and learn the power of Hess's Law and how to use Germain Hess's concept of the standard enthalpy of formation to calculate exactly how much heat is produced by any chemical reaction.
Chapters:
State Function
Path-Dependent Function
Enthalpy
Bonds are Energy
Colorimetry
Hess' Law
Standard Enthalpy of Formation
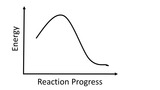
This module covers four areas of enthalpy:1- introduction to enthalpy and reaction-energy diagrams; define exothermic and endothermic2- Thermochemical expressions and manipulating enthalpy3- Hess's Law4- Enthalpies of formation to find enthalpy of reaction
Students explore material properties by applying some basic principles of heat transfer. They use calorimeters to determine the specific heat of three substances: aluminum, copper and another of their choice. Each substance is cooled in a freezer and then placed in the calorimeter. The temperature change of the water and the substance are used in heat transfer equations to determine the specific heat of each substance. The students compare their calculated values with tabulated data.
You and a friend are hiking the Appalachian Trail when a storm comes through. You stop to eat, but find that all available firewood is too wet to start a fire. From your Chem 106 class, you remember that heat is given off by some chemical reactions; if you could mix two solutions together to produce an exothermic reaction, you might be able to cook the food you brought along for the hike. Luckily, being the dedicated chemist that you are, you never go anywhere without taking along a couple chemical solutions called X and Y just for times like this. The Virtual Lab contains solutions of compounds X and Y of various concentrations.

This resource is a video abstract of a research paper created by Research Square on behalf of its authors. It provides a synopsis that's easy to understand, and can be used to introduce the topics it covers to students, researchers, and the general public. The video's transcript is also provided in full, with a portion provided below for preview:
"As materials scientists know well, one reliable way to make strong metals even stronger is to shrink their already-tiny crystalline grains. It’s a time-tested technique that’s made today’s cars, planes, and armor safer than ever. But at the nanoscale, grains are notoriously fickle. Their strong tendency to grow makes it nearly impossible for researchers to chase higher levels of strength. But that could soon change. A new computer model developed by researchers from MIT shows how nano-sized grains might be stabilized in metal alloys. Their findings could provide the blueprint for constructing harder and stronger metals. Alloying one metal with another is one technique that has helped researchers push grain sizes to smaller and smaller scales—thanks to a process known as segregation. As the grains in a metal shrink, the addition of a small amount of an alloying metal segregate, or adhere, to the boundaries between different grains..."
The rest of the transcript, along with a link to the research itself, is available on the resource itself.

This subject deals primarily with equilibrium properties of macroscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and rates of chemical reactions.
Acknowledgements
The material for 5.60 has evolved over a period of many years, and therefore several faculty members have contributed to the development of the course contents. The following are known to have assisted in preparing the lecture notes available on OpenCourseWare: Emeritus Professors of Chemistry: Robert A. Alberty, Carl W. Garland, Irwin Oppenheim, John S. Waugh. Professors of Chemistry: Moungi Bawendi, John M. Deutch, Robert W. Field, Robert G. Griffin, Keith A. Nelson, Robert J. Silbey, Jeffrey I. Steinfeld. Professor of Bioengineering and Computer Science: Bruce Tidor. Professor of Chemistry, Rice University: James L. Kinsey. Professor of Physics, University of Illinois: Philip W. Phillips.
This subject deals primarily with equilibrium properties of macroscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and rates of chemical reactions.
Acknowledgements
The material for 5.60 has evolved over a period of many years, and therefore several faculty members have contributed to the development of the course contents. The following are known to have assisted in preparing the lecture notes available on OpenCourseWare: Emeritus Professors of Chemistry: Robert A. Alberty, Carl W. Garland, Irwin Oppenheim, John S. Waugh. Professors of Chemistry: Moungi Bawendi, John M. Deutch, Robert W. Field, Robert G. Griffin, Keith A. Nelson, Robert J. Silbey, Jeffrey I. Steinfeld. Professor of Bioengineering and Computer Science: Bruce Tidor. Professor of Chemistry, Rice University: James L. Kinsey. Professor of Physics, University of Illinois: Philip W. Phillips.