
10 Results


Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.
- Subject:
- Biology
- Life Science
- Material Type:
- Full Course
- Provider:
- Rice University
- Provider Set:
- OpenStax College
- Date Added:
- 08/22/2012
- Subject:
- Biology
- Life Science
- Material Type:
- Unit of Study
- Provider:
- Rice University
- Provider Set:
- OpenStax College

- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017
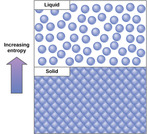
By the end of this section, you will be able to:Discuss the concept of entropyExplain the first and second laws of thermodynamics
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Author:
- Tina B. Jones
- Date Added:
- 08/15/2019

By the end of this section, you will be able to:Discuss the concept of entropyExplain the first and second laws of thermodynamics
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

This Lesson provides two different activities that require students to measure energy outputs and inputs to determine the efficiency of conversions and simple systems. One of the activities includes Lego motors and accomplishing work. The other investigates energy for heating water. They learn about by products of energy conversions and how to improve upon efficiency. The teacher can choose to use either of these or both of these. The calculations in the water heating experiment are more complicated than in the Lego motor activity. Thus, the heating activity is suitable for older students, only the Lego motor activity suitable for younger students.
- Subject:
- Applied Science
- Engineering
- Material Type:
- Activity/Lab
- Lesson Plan
- Provider:
- TeachEngineering
- Provider Set:
- TeachEngineering
- Author:
- Jan DeWaters
- Nate Barlow
- Susan Powers
- Date Added:
- 09/18/2014
Life is chaos and the universe tends toward disorder. But why? If you think about it, there are only a few ways for things to be arranged in an organized manner, but there are nearly infinite other ways for those same things to be arranged. Simple rules of probability dictate that it's much more likely for stuff to be in one of the many disorganized states than in one of the few organized states. This tendency is so unavoidable that it's known as the 2nd Law of Thermodynamics. Obviously, disorder is a pretty big deal in the universe and that makes it a pretty big deal in chemistry - it's such a big deal that scientists have a special name for it: entropy. In chemistry, entropy is the measure of molecular randomness or disorder. For the next thirteen minutes, Hank hopes you will embrace the chaos as he teaches you about entropy.
Chapters:
Second Law of Thermodynamics
Entropy
DEMONSTRATION!
BA(OH)2•8H2O+NH4Ci
J.W. Gibbs & Gibbs Free Energy
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Lecture
- Provider:
- Complexly
- Provider Set:
- Crash Course Chemistry
- Date Added:
- 07/02/2013
Doelstelling van dit college is een introductie te geven in de theorie van de Thermodynamica, een van de fundamentele werktuigbouwkunde vakken. De Thermodynamica behandelt energie vraagstukken en relaties tussen de eigenschappen van materialen. In dit college wordt voor een ingenieurs aanpak van de Thermodynamica gekozen: onderwerp van studie zijn systemen en hun interactie met de omgeving. Naast gesloten systemen krijgen open systemen veel aandacht. Thermodynamica wordt, in combinatie met stromingsleer en warmte- en stofoverdracht, ingezet om bijvoorbeeld automotoren, turbines, compressoren, pompen, elektriciteit opwekkinginstallaties, cryogenische-, koel- en klimaat-installaties en duurzame energieconversie installaties te analyseren en ontwerpen. De beginselen van de Thermodynamica maken het mogelijk om de ontwerpen van energie gerelateerde werktuigbouwkundige apparaten en systemen te optimaliseren voor het betreffende doel.
- Subject:
- Applied Science
- Engineering
- Material Type:
- Assessment
- Homework/Assignment
- Lecture
- Lecture Notes
- Reading
- Provider:
- Delft University of Technology
- Provider Set:
- TU Delft OpenCourseWare
- Author:
- B.J. Boersma
- Date Added:
- 10/26/2014

This subject deals primarily with equilibrium properties of macroscopic and microscopic systems, basic thermodynamics, chemical equilibrium of reactions in gas and solution phase, and macromolecular interactions.
- Subject:
- Applied Science
- Biology
- Chemistry
- Engineering
- Life Science
- Physical Science
- Physics
- Material Type:
- Full Course
- Provider Set:
- MIT OpenCourseWare
- Author:
- Bawendi, Moungi
- Field, Robert
- Griffith, Linda
- Hamad-Schifferli, Kim
- Date Added:
- 09/01/2005