
47 Results

In this video segment, the ZOOM cast demonstrates how to use cabbage juice to find out if a solution is an acid or a base.
- Subject:
- Chemistry
- Physical Science
- Physics
- Material Type:
- Activity/Lab
- Provider:
- PBS LearningMedia
- Provider Set:
- PBS Learning Media: Multimedia Resources for the Classroom and Professional Development
- Author:
- National Science Foundation
- WGBH Educational Foundation
- Date Added:
- 02/20/2004

Both of these lessons are classroom activities that require students to build models that display understanding of atoms and molecules. One lesson is structured while the other is guided.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- Science Education Resource Center (SERC) at Carleton College
- Provider Set:
- Pedagogy in Action
- Author:
- Carrie Robatcek
- Date Added:
- 08/10/2012

Biology is designed for multi-semester biology courses for science majors. It is grounded on an evolutionary basis and includes exciting features that highlight careers in the biological sciences and everyday applications of the concepts at hand. To meet the needs of today’s instructors and students, some content has been strategically condensed while maintaining the overall scope and coverage of traditional texts for this course. Instructors can customize the book, adapting it to the approach that works best in their classroom. Biology also includes an innovative art program that incorporates critical thinking and clicker questions to help students understand—and apply—key concepts.
- Subject:
- Biology
- Life Science
- Material Type:
- Full Course
- Provider:
- Rice University
- Provider Set:
- OpenStax College
- Date Added:
- 08/22/2012
- Subject:
- Biology
- Life Science
- Material Type:
- Unit of Study
- Provider:
- Rice University
- Provider Set:
- OpenStax College
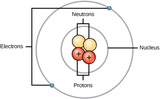
By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Author:
- Tina B. Jones
- Date Added:
- 08/26/2019

By the end of this section, you will be able to:Define matter and elementsDescribe the interrelationship between protons, neutrons, and electronsCompare the ways in which electrons can be donated or shared between atomsExplain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

- Subject:
- Applied Science
- Biology
- Life Science
- Material Type:
- Module
- Date Added:
- 07/10/2017

Blue Coral Periodic Table is your quick guide to all 118 elements. Swipe and tap your way across the table for quick stats on each element. Dive deeper and recolor the table with patterns based off properties such as boiling point, melting point, and atomic radius.
View the atomic model for each element and see how the electron configuration changes as you move from element to element.
Blue Coral Periodic Table is fully responsive in the web browser for large and small devices in both horizontal and vertical orientations.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Diagram/Illustration
- Interactive
- Provider:
- Blue Coral Learning
- Date Added:
- 12/06/2017

Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Simulation
- Provider:
- University of Colorado Boulder
- Provider Set:
- PhET Interactive Simulations
- Author:
- Jack Barbera
- John Blanco
- Kathy Perkins
- Kelly Lancaster
- Patricia Loeblein
- Robert Parson
- Sam Reid
- Suzanne Brahmia
- Date Added:
- 07/13/2011

This course is an OER section developed by Dr. Ara Kahyaoglu for Bergen Community College. The primary text was developed for the Saylor Academy and is modified to better serve the course objectives for BCC students.Chapter 5 - Periodic TrendsLearning Objective 1. Be able to state how certain properties of atoms vary based on their relative position on the periodic table.

This course is an OER section developed by Dr. Ara Kahyaoglu for Bergen Community College. The primary text was developed for the Saylor Academy and is modified to better serve the course objectives for BCC students.Chapter 5 - Periodic TrendsLearning Objective 1. Be able to state how certain properties of atoms vary based on their relative position on the periodic table.

In this activity, students will learn the location of the following categories on the periodic table while creating their own version including a key.Categories Included:Alkali MetalsAlkaline Earth MetalsHalogensNoble GasesMetalsNonmetalsMetalloidsTransition MetalsInner Transition MetalsThe Soft Chalk Activity includes interactive checks throughout and includes information on valence electrons and determining groups and periods for elements.This activity also includes a formative assessment that students could take when they are done.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Homework/Assignment
- Interactive
- Author:
- Julie Buerman
- Date Added:
- 07/28/2020

Matching game, including matching common ionic charges and ions and the compounds they form and naming of greek prefixes for covalent molecules.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- Science Education Resource Center (SERC) at Carleton College
- Provider Set:
- Pedagogy in Action
- Author:
- Dustin Redinius
- Date Added:
- 08/10/2012

This activity provides a demonstration and lab exploration of one of the main "building blocks" of the periodic table of elements: chlorine. During the lab, students compare physical and chemical properties of chlorine compounds.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Simulation
- Provider:
- Science Education Resource Center (SERC) at Carleton College
- Provider Set:
- Pedagogy in Action
- Author:
- Kate Tinguely
- Date Added:
- 08/16/2012

This interactive, scaffolded activity allows students to build an atom within the framework of a newer orbital model. It opens with an explanation of why the Bohr model is incorrect and provides an analogy for understanding orbitals that is simple enough for grades 8-9. As the activity progresses, students build atoms and ions by adding or removing protons, electrons, and neutrons. As changes are made, the model displays the atomic number, net charge, and isotope symbol. Try the "Add an Electron" page to build electrons around a boron nucleus and see how electrons align from lower-to-higher energy. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology. The Concord Consortium develops deeply digital learning innovations for science, mathematics, and engineering. The models are all freely accessible. Users may register for additional free access to capture data and store student work products.
- Subject:
- Applied Science
- Chemistry
- Physical Science
- Physics
- Technology
- Material Type:
- Lesson
- Provider:
- Concord Consortium
- Provider Set:
- Concord Consortium Collection
- Author:
- The Concord Consortium
- Date Added:
- 05/06/2011
This interactive activity from ChemThink takes a closer look at a covalent bond--how it is formed and how the sharing of two electrons can keep atoms together.
- Subject:
- Chemistry
- Physical Science
- Physics
- Material Type:
- Activity/Lab
- Interactive
- Provider:
- PBS LearningMedia
- Provider Set:
- PBS Learning Media: Multimedia Resources for the Classroom and Professional Development
- Author:
- National Science Foundation
- WGBH Educational Foundation
- Date Added:
- 08/09/2007

This activity is set as an introduction to the periodic table. Students will be organizing and categorizing objects or ideas of their own choosing.
- Subject:
- Educational Technology
- Material Type:
- Lesson Plan
- Author:
- Everett Charlson
- Date Added:
- 06/16/2018

This is a lab activity where the students group the given elements as metals, nonmetals or metalloids.
- Subject:
- Chemistry
- Physical Science
- Material Type:
- Activity/Lab
- Assessment
- Lesson Plan
- Provider:
- Science Education Resource Center (SERC) at Carleton College
- Provider Set:
- Pedagogy in Action
- Author:
- Lakshmi Karthikeyan
- Date Added:
- 12/09/2011

Students learn about the periodic table and how pervasive the elements are in our daily lives. After reviewing the table organization and facts about the first 20 elements, they play an element identification game. They also learn that engineers incorporate these elements into the design of new products and processes. Acting as computer and animation engineers, students creatively express their new knowledge by creating a superhero character based on of the elements they now know so well. They will then pair with another superhero and create a dynamic duo out of the two elements, which will represent a molecule.
- Subject:
- Applied Science
- Chemistry
- Engineering
- Physical Science
- Material Type:
- Activity/Lab
- Provider:
- TeachEngineering
- Provider Set:
- TeachEngineering
- Author:
- Brian Kay
- Denise W. Carlson
- Lauren Cooper
- Malinda Schaefer Zarske
- Megan Podlogar
- Date Added:
- 10/14/2015